To address the issue of surface and groundwater contamination, researchers from University of Tabriz (Iran), in collaboration with Istanbul Technical University (Turkey), Farhangian University (Iran), and Al-Farabi Kazakh National University (Kazakhstan), have developed an innovative solution based on ZnFe₂O₄@ZIF-67 composite nanostructures.
According to the report, this nanocomposite—synthesized through hydrothermal and solvothermal methods—demonstrated outstanding efficiency in activating peroxydisulfate (PDS) and enabling the rapid degradation of furazolidone through a triple ionic synergistic system. The findings reveal that the combination of ultrasound waves and this nanostructure can eliminate a significant portion of this pharmaceutical pollutant from real water samples within a short time, paving the way for industrial applications in water purification technologies.
Contamination of surface and groundwater with chemical and pharmaceutical substances has become one of the most pressing environmental concerns in recent decades. Population growth, rapid industrialization, and excessive exploitation of natural resources have contributed to the release of large amounts of pollutants into the aquatic ecosystem. Among these pollutants, pharmaceutical compounds—particularly antibiotics— pose a serious challenge due to their high stability and resistance to natural degradation processes. One such compound is furazolidone, a member of the nitrofuran family, which has long been used as an antibacterial and antiparasitic drug in both human and veterinary medicine. Although therapeutically effective, studies have shown that furazolidone exhibits mutagenic, genotoxic, and potentially carcinogenic properties. As a result, its use has been banned in many countries; however, traces of the drug are still detected in aquatic environments in certain regions.
The urgent need for efficient methods to remove furazolidone and similar drugs has driven researchers toward advanced oxidation processes (AOPs)—innovative technologies that generate highly reactive oxygen radicals capable of breaking down persistent organic pollutants. Among these, sonocatalysis—which combines ultrasound irradiation with heterogeneous catalysts—has attracted significant attention. Ultrasound waves cause cavitation phenomena, leading to localized zones of extremely high temperature and pressure, which promote the formation of reactive radicals and accelerate chemical reactions. When coupled with advanced catalytic materials, this effect can substantially enhance pollutant degradation efficiency.
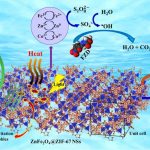
In this context, a team of scientists from the University of Tabriz and Farhangian University, in collaboration with partners from Turkey and Kazakhstan, has designed and synthesized a novel nanostructure that shows exceptional performance in removing furazolidone from contaminated water. This structure, known as ZnFe₂O₄@ZIF-67, combines zinc ferrite (ZnFe₂O₄) with zeolitic imidazolate framework (ZIF-67). Each component offers unique advantages: zinc ferrite contributes magnetic properties and chemical stability, while ZIF-67 provides a high surface area, porous structure, and strong oxidative capability. Their integration into a heterostructure enables synergistic effects, enhancing the availability of active sites for oxidation reactions.
To synthesize the nanostructure, researchers employed hydrothermal and solvothermal techniques, resulting in samples with high crystallinity, narrow band gap, and large specific surface area. Experimental results showed that applying the nanostructure in the presence of peroxydisulfate (PDS) and ultrasound waves rapidly activated the oxidant and generated strong radicals such as sulfate radicals (SO₄•–), hydroxyl radicals (•OH), and singlet oxygen (¹O₂). Together with surface redox reactions, these radicals led to 94% degradation of furazolidone under optimal conditions.
Tests demonstrated that under conditions of 20 mM PDS and 0.4 g/L of the ZnFe₂O₄@ZIF-67 nanocomposite, nearly all furazolidone molecules were decomposed within 60 minutes. Another important finding was the stability and reusability of the nanostructure, which maintained its catalytic performance even after multiple reaction cycles. Using liquid chromatography–mass spectrometry (LC–MS), researchers identified the possible degradation pathway of furazolidone and confirmed that the intermediate products were less toxic than the original compound.
A distinctive feature of this study is the use of a “triple ionic synergistic system”, involving redox cycles of Zn²⁺/Zn, Fe³⁺/Fe²⁺, and Co³⁺/Co²⁺ ions. These redox interactions facilitated charge transfer and enhanced radical generation, leading to a significant improvement in overall degradation efficiency. Thus, the innovative ZnFe₂O₄@ZIF-67 nanocomposite not only achieved high performance in removing pharmaceutical pollutants but also demonstrated excellent stability and industrial applicability.
This achievement could mark a turning point in modern water treatment technologies, as many common drugs—such as antibiotics and other resistant organic compounds—are difficult to remove through conventional purification methods. Nanotechnology-based systems combining sonocatalysis and oxidant activation may open new avenues for developing high-efficiency, cost-effective industrial water treatment plants.
The researchers emphasized that this method has proven effective not only at the laboratory scale but also in real water samples contaminated with pharmaceuticals. Therefore, with continued research and optimization of operational conditions, this approach holds strong potential for commercialization, contributing significantly to public health protection and environmental sustainability.